EndoDrill® – powered rotating needle provides high-quality core biopsies
EndoDrill® products are designed to obtain high-quality tissue samples, aiming to improve diagnostics for several cancer types, including stomach, pancreatic, liver, lung, and bladder cancer. This innovative technology is helping redefine the growing field of endoscopic ultrasound-guided tissue acquisition in cancer diagnostics. The powered, high-speed rotational EndoDrill® needle technique enables true core biopsies, addressing the limitations of today’s standard manual needles.
EndoDrill® is designed for ease of use and consists of a sterile, single-use EndoDrill® Biopsy Instrument paired with the reusable EndoDrill® Drive System. The product received FDA 510(k) clearance in the U.S. in 2023, followed by CE certification under the MDR in Europe in 2024. Commercial launch will begin in the U.S. in 2025, in collaboration with distribution partner TaeWoong Medical USA.
1. Swahn F, Glavas R, Hultin L, Wickbom M. The advent of the first electric driven EUS-guided 17 gauge core needle biopsy – A pilot study on subepithelial lesions. Scandinavian Journal of Gastroenterology. 2024;59(7):852–858.
2. Mendoza Ladd A, Alsamman A, Meiklejohn K, m.fl. Initial Experience With The Transmural Use Of A New Endoscopic Ultrasound Electric Core Needle Biopsy Device: A Case Series. Endoscopy International Open. 2024.
3. Oliver M, Kabir K, Ognjanovski E, Barawi M. Exploring the Impact of the First Ever Endoscopic Ultrasound-Guided Motorized Fine Needle Aspiration (“EndoDrill”) Liver Biopsy and Additional Cases: Case Vignette. Presentation vid 4th Annual Michigan GI Society Conference, 2025.
 One Swedish clinical study1 and two U.S. case series2,3 demonstrated 100 % diagnostic accuracy for EndoDrill® GI in multiple GI indications.
One Swedish clinical study1 and two U.S. case series2,3 demonstrated 100 % diagnostic accuracy for EndoDrill® GI in multiple GI indications.
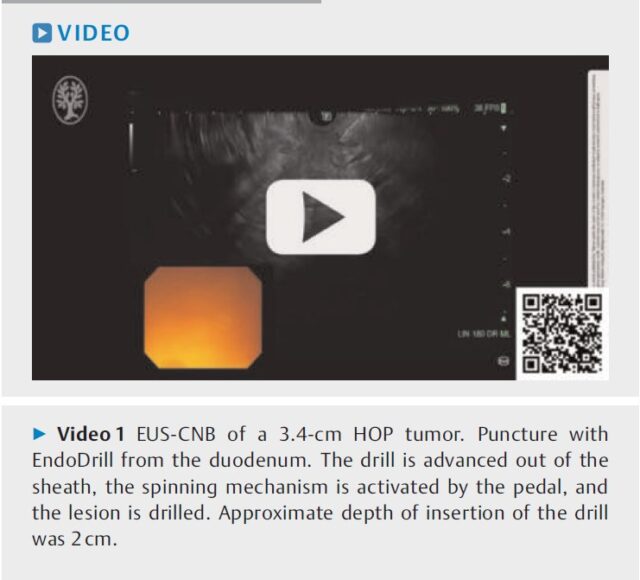
Invest 40 seconds and learn the basics about the world’s first FDA-cleared electric biopsy instrument for endoscopic ultrasound.

BiBB is a cancer diagnostics company that develops and manufactures EndoDrill®, a patented product line of powered endoscopic biopsy instruments.

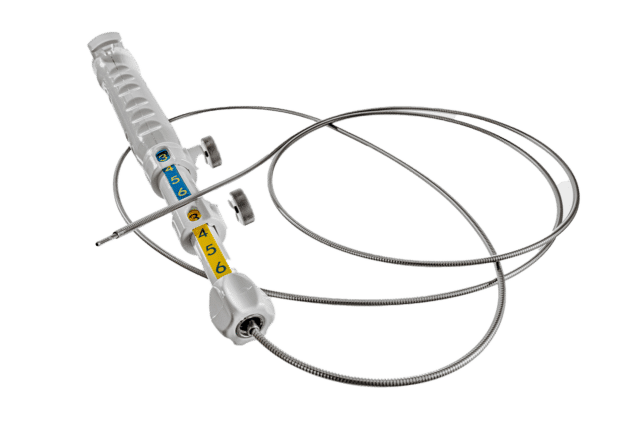
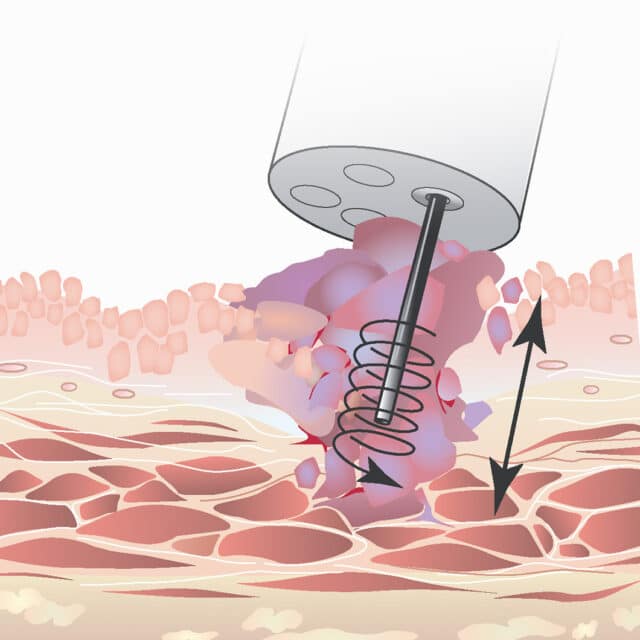
Unlike conventional manual needle instruments, EndoDrill® features a rotating drill-tip cylinder. This design enables deep, precise tissue sampling. One or more solid core biopsies are extracted – providing the tissue architecture required for complete histological diagnosis, staging, and genetic analysis.
The EndoDrill® product line includes single-use biopsy instruments with a flexible drill-tip cylinder and a reusable drive system consisting of a motor unit, foot pedal, and drive cable. EndoDrill® is specifically developed to collect tissue samples of the highest quality during ultrasound-guided endoscopic procedures (EUS/EBUS).

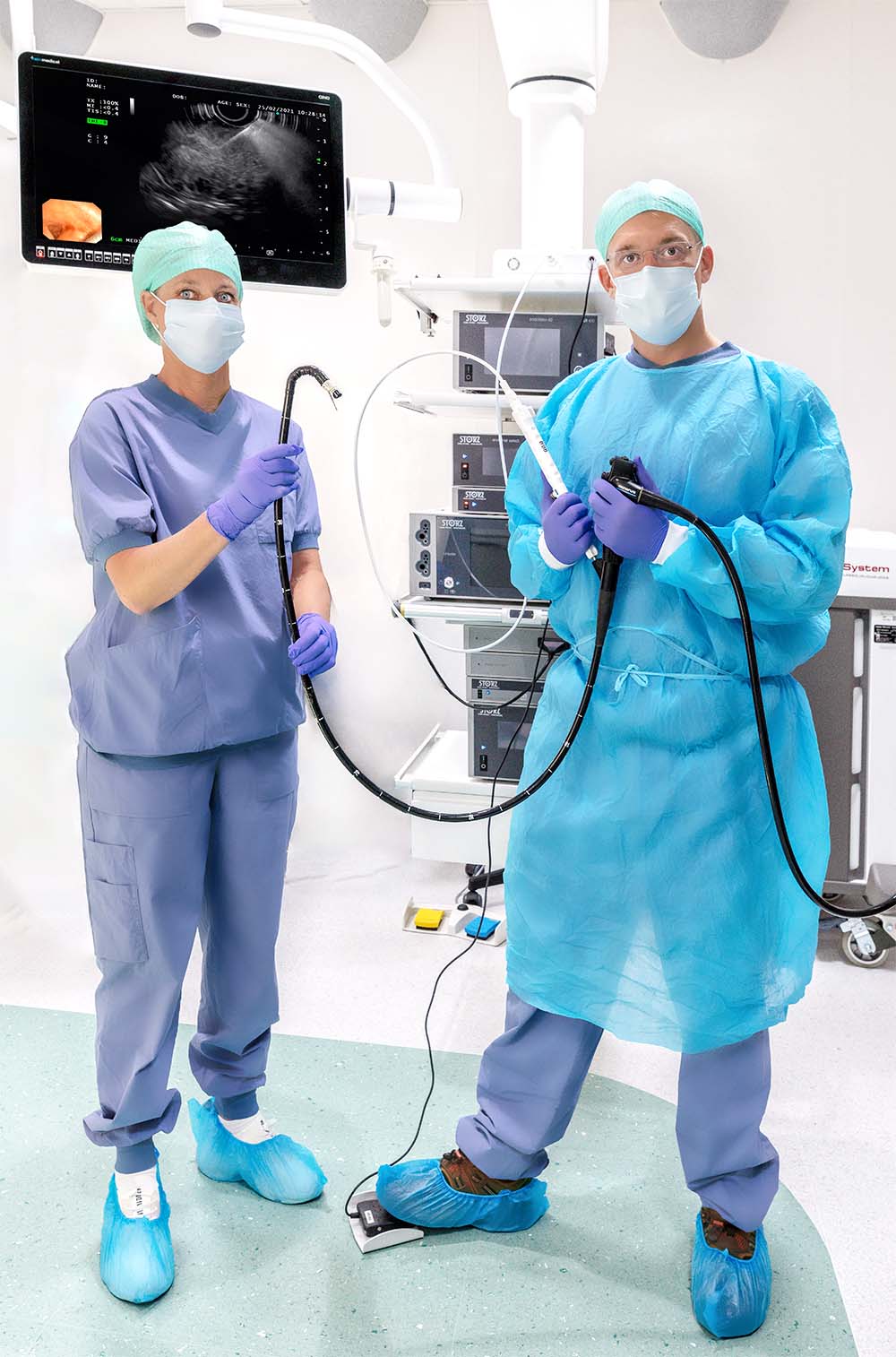
Click the icons in the image to learn more about EndoDrill®.
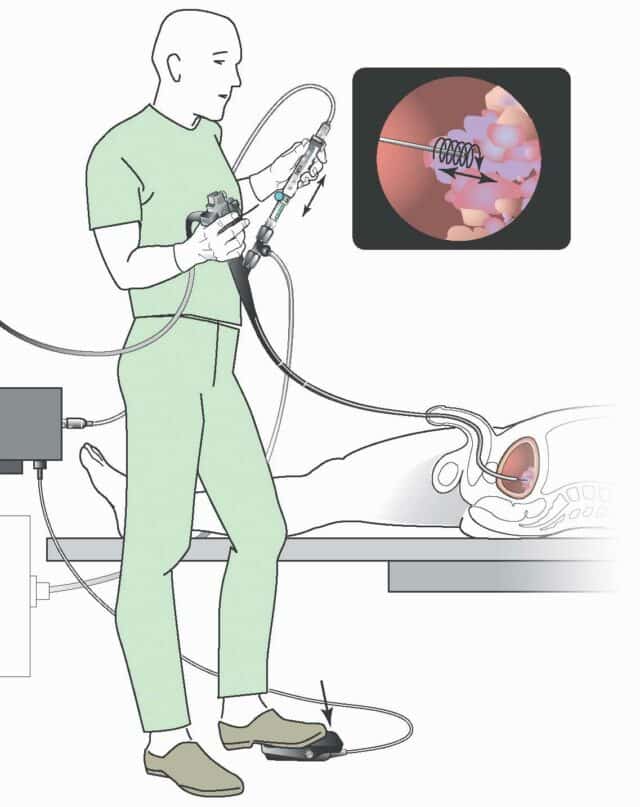
A compact motor unit connects to the EndoDrill® Drive Cable and is operated via the EndoDrill® Foot Pedal. The motor unit generates a constant rotational speed, which is transferred through the Drive Cable to the drill tip cylinder of the single-use EndoDrill® Biopsy Instrument.
EndoDrill®’s rotating drill tip cylinder cuts solid core biopsies to maximize diagnostic accuracy. The flexible drill tip cylinder is protected by a surrounding outer sheath.
The EndoDrill® Drive Cable transmits rotation from the EndoDrill® Motor Unit to the drill tip cylinder of the EndoDrill® Biopsy Instrument.
The handle includes components for adjusting and setting the desired extension of the sheaths. Two flexible sheaths are connected to the handle: an inner sheath containing the drill tip cylinder, and a protective outer sheath.
The EndoDrill® Foot Pedal starts and stops the rotation generated by the motor unit, giving the operator full control during the procedure.
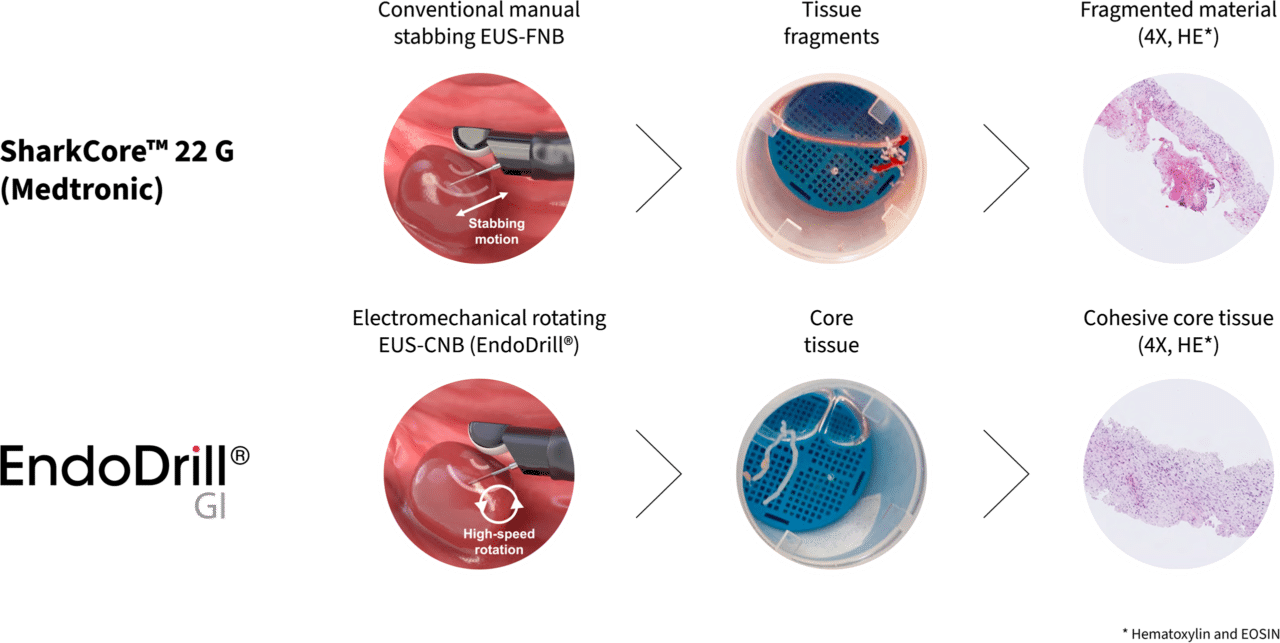
Today’s manual EUS/EBUS needle instruments are inserted into tumors using a repeated stabbing motion, and detached cells and tissue fragments adhere to the tip of the needle. The method requires skilled endoscopists for sampling and experienced pathologists for evaluation. To increase the chances of the fragmented cell material leading to a diagnosis, it is not uncommon to use a variety of supplemental methods, such as having a pathologist in the operating room for Rapid On-Site Evaluation (ROSE), centrifuging the sample (i.e., cellblock), taking multiple samples at different sites, and using different techniques to aspirate the cell sample.
EndoDrill® is designed to simplify the diagnostic process. Instead of manual sampling through repeated stabbing, tissue is collected using a powered, user-friendly drilling technique. In the majority of cases, a single needle pass is sufficient to obtain a diagnostic result*.
*Mendoza Ladd A, Pham KDC, Pavic T, Glavas R, Swahn F. EndoDrill® core needle biopsy: preliminary multicenter experience. Endoscopy. 2025;57(Suppl 2):182–3. doi:10.1055/s-0045-1805456.
The new era of personalised cancer treatment requires high-quality core biopsies for histological diagnosis and genetic analysis.

The endoscopist drills out core biopsy samples in which the tissue architecture is preserved, often with low or no blood contamination. Sampling with EndoDrill® may become more reproducible and standardized (i.e., less dependent on the experience and skill of the endoscopist).
Another very important and unique feature is the flexible design, which enables sampling at a highly angulated position. This means that high-quality samples can be taken from very hard-to-reach tumours, which are often technically challenging to access with today’s more rigid biopsy needles.
|
EndoDrill® |
Current standard of care |
|
|---|---|---|
| Acquired tumor tissue quality |
Solid core biopsies with preserved histological architecture resulting in high diagnostic accuarcy2,3,4 |
It is not uncommon for FNA/FNB to yield blood-tinged material containing detached cells and tissue fragments of varying quality |
| Tissue samples adequate for | For both histology and genetic analysis1,2,3 | Varies widely depending e.g. operator experience from only cytological diagnosis to a more complete diagnosis |
| Time required | Potentially shorter procedure with powered rotation, fewer needle passes/stabs, fewer samples required3, 4 | Time consuming with manual, multiple needle passes/stabs; many samples required |
| Flexibility of the needle instrument | Ultra-flexible instrument – works even with a highly angled endoscope |
Manual tissue penetration requires a more rigid needle instrument, which may perform poorly with a highly curved endoscope |
| Need for supplementary techniques | High quality biopsies obtained without additionally laboratory techniques/ROSE1,2,3 | Often requires additional resource-intensive techniques, e.g. ROSE and cell block, to increase diagnostic yield |
1. Swahn et al., 2022, EndoDrill® Model X Biopsy Instrument, The Advent of the First EUS Guided 17 Gauge Core Needle Biopsy, Poster session presented at DDW, San Diego.
2. Swahn et al., 2024, The advent of the first electric driven EUS-guided 17 gauge core needle biopsy – A pilot study on subepithelial lesions. Scandinavian Journal of Gastroenterology, 1–7. https://doi.org/10.1080/00365521.2024.2336611
3. Mendoza Ladd A et al. Initial Experience With The Transmural Use Of A New Endoscopic Ultrasound Electric Core Needle Biopsy Device: A Case Series. Endoscopy International Open 2024. doi: 10.1055/a-2427-2311
4. Mendoza Ladd A, Pham KDC, Pavic T, Glavas R, Swahn F. EndoDrill® core needle biopsy: preliminary multicenter experience. Endoscopy. 2025;57(Suppl 2):182–3. doi:10.1055/s-0045-1805456.
Using the new EndoDrill® high-quality core needle biopsy (CNB) at the first examination makes it possible to achieve the goal of a treatment-determining diagnosis immediately, while avoiding resource-intensive methods and repeated sampling. Early definitive diagnosis with histological and genetic information enables personalised treatment to be initiated immediately, which can save both lives and healthcare resources.
From EUS tissue sampling with fine-needle aspiration of cells (EUS-FNA) of the 2000s to fine-needle biopsy (EUS-FNB) of tissue fragments of the 2010s, introduction of the powered EndoDrill® is the 2020s’ solution for the sampling of high-quality core needle biopsies (EUS-CNB). It is a long-awaited improvement in diagnostics for many types of cancer, with the potential to eventually establish a new standard of care for endoscopic sampling.

BiBB is currently developing and manufacturing three product variants of EndoDrill® that can be used for endoscopic tissue sampling in six of the ten most common forms of cancer. The EndoDrill® products are compatible with endoscopes from the major manufacturers.
Each variant is tailored to the specific requirements and clinical environments of its intended application. EndoDrill® GI (gastrointestinal tract) and EndoDrill® EBUS (airways/lung) are used with ultrasound endoscopes, while EndoDrill® URO (urinary tract) is designed for use with conventional cystoscopes.![]()
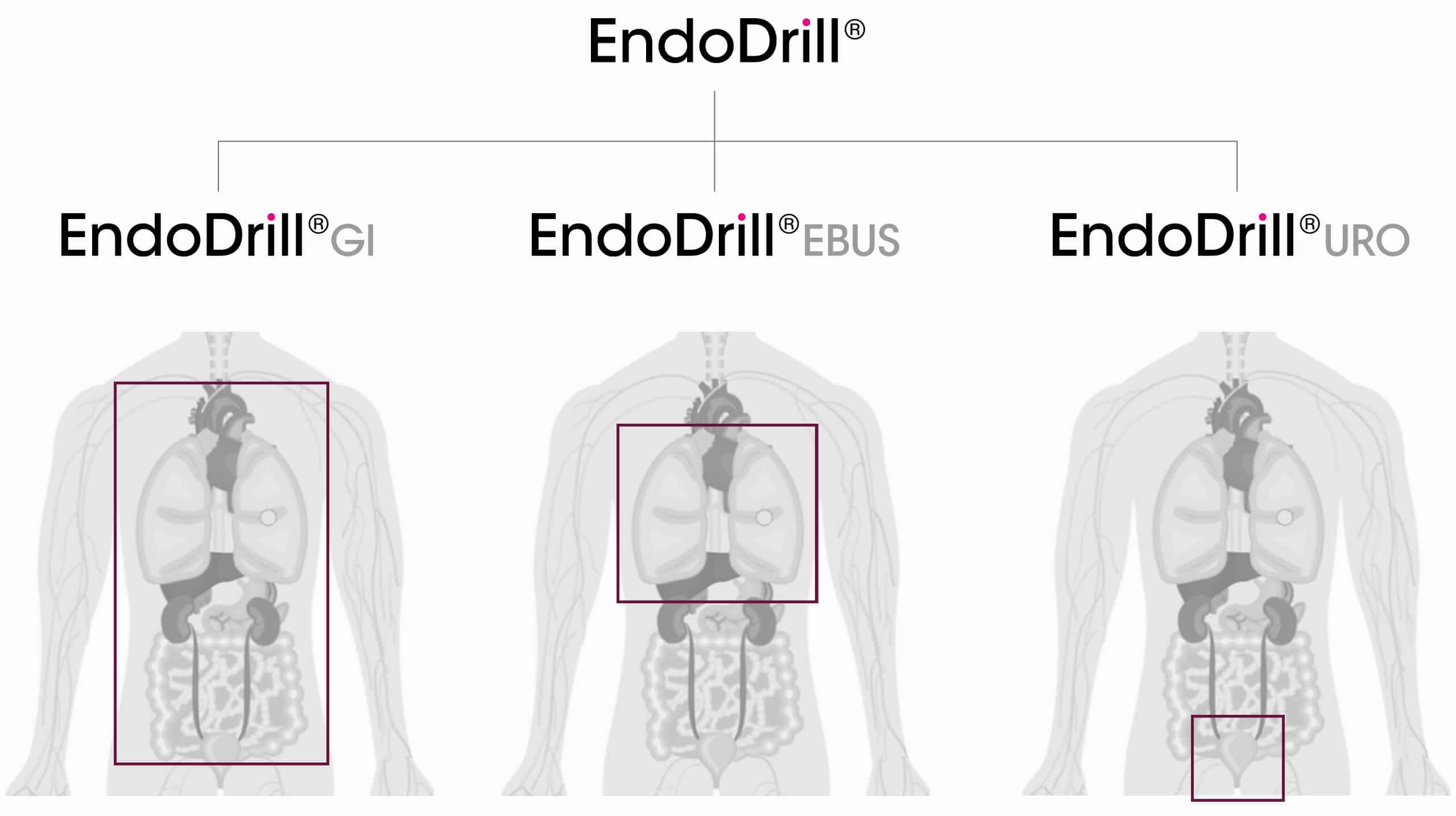
Learn more about the different variants of EndoDrill® by tapping the plus-icons in the image.
is used for ultrasound-guided (EUS) sampling of organs in and around the gastrointestinal tract, such as the stomach, pancreas, liver, and lymph nodes
is used in the respiratory tract in endobronchial ultrasound (EBUS) tissue sampling for the diagnosis and staging of lung cancer.
is used with a standard cystoscope to obtain tissue samples for the diagnosis and staging of muscle-invasive bladder cancer during the initial endoscopic procedure.
EndoDrill® GI is used for endoscopic ultrasound (EUS) tissue sampling for all indications in the gastrointestinal tract, such as in the pancreas, stomach, oesophagus, lymph nodes and liver.
 In the majority of patient cases reported in published clinical studies¹,², EndoDrill® GI obtained diagnostic core biopsies with only a single needle pass
In the majority of patient cases reported in published clinical studies¹,², EndoDrill® GI obtained diagnostic core biopsies with only a single needle pass
1. Mendoza Ladd A, Alsamman A, Meiklejohn K, et al Initial Experience With The Transmural Use Of A New Endoscopic Ultrasound Electric Core Needle Biopsy Device: A Case Series. Endoscopy International Open. 2024.
2. Mendoza Ladd A, Pham KDC, Pavic T, Glavas R, Swahn F. EndoDrill® Core Needle Biopsy: Preliminary Multicenter Experience. Endoscopy. 2025;57(Suppl 2):182–183.
The series of images shows EndoDrill®s demonstrated ability to take treatment-determining tissue
samples of the highest possible quality
Download leaflet for EndoDrill® GI
Are you representing a U.S. hospital interested in EndoDrill® GI? Click below to reach our U.S. partner, TaeWoong Medical USA, and connect with a sales representative.

Learn more at TaeWoong Medical USA
Instructions for use including safety and performance information relevant to the user or any other person accompanies the product or provided upon request. Please contact BiBBInstruments at support@bibbinstruments.com.
EndoDrill® EBUS is used in the airways during endobronchial ultrasound (EBUS) sampling for the diagnosis and staging of lung cancer. As more and more targeted treatments become available, demands on the quality of diagnostic tissue samples are increasing, and today’s fine-needle instruments do not fully meet expectations. EndoDrill® EBUS is designed to meet the new requirements for histopathological and genetic analyses. With the right diagnosis, the right treatment can be initiated –even in severe cases
Aiming to revolutionize lung cancer diagnostics by enabling both histopathological and genetic analyses.
EndoDrill® URO is used with a standard cystoscope for sampling of muscle-invasive bladder cancer (MIBC). The purpose of EndoDrill® URO is to take tissue samples of deep-growing bladder tumors for the first time already at the initial cystoscopy. With an earlier diagnosis, today’s invasive surgical procedures (TURB) could be avoided and the treatment of patients with MIBC could be started earlier. An initial pilot study shows that EndoDrill® URO safely can take treatment-based samples earlier in the care chain if MIBC is suspected.
Potential paradigm shift for the diagnosis of muscle-invasive bladder cancer (MIBC).
BiBB and its clinical partners have conducted preclinical comparisons, a clinical pilot study, a clinical case series, and a retrospective multicenter study using EndoDrill® GI. Overall, EndoDrill® GI obtains high-quality core biopsies, often with the first needle pass. In cases where EndoDrill® GI has been compared with leading competitors (EUS-FNB devices), results show that EndoDrill® provides tissue samples of superior diagnostic quality. BiBB is continuously working on clinical follow-up to strengthen the clinical evidence base.
In the autumn of 2020, the first clinical study, EDMX01, was initiated at three Swedish university hospitals for sampling hard-to-diagnose upper gastrointestinal cancer (SEL tumours).
The analysis showed that EndoDrill® safely collected high-quality tissue samples, known as core biopsies, even in comparison with leading fine needle biopsy instruments (EUS-FNB).
The study was presented in May 2022 at the DDW congress in San Diego, USA, and published in the peer reviewed Scandinavian Journal of Gastroenterology in March 2024.
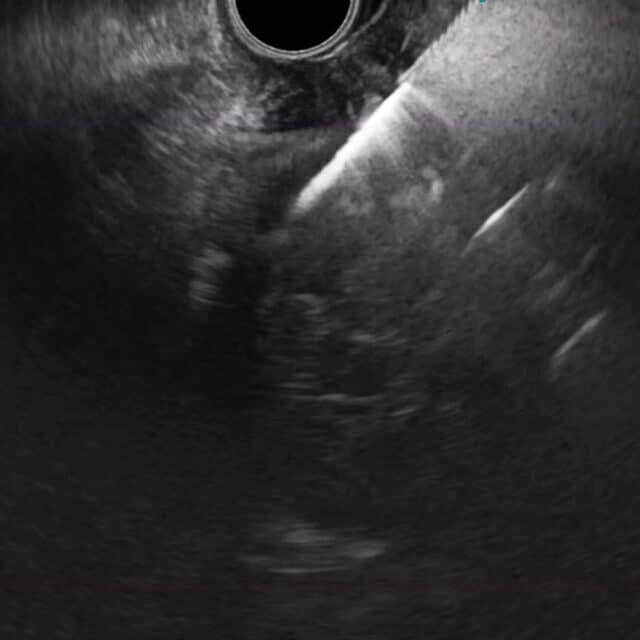
The ultrasound image clearly shows when EndoDrill® GI is drilled into a submucosal tumour.
The series of images below from case 2 of study EDMX01 shows the difference in tissue sample quality when biopsies are taken from the same tumour with EndoDrill® GI and a leading EUS-FNB instrument. Large and solid tissue samples contain more information and improve diagnostic accuracy.
Link to the scientific publication in Scandinavian Journal of Gastroenterology
After receiving FDA 510(k) clearance and CE marking (MDR) for EndoDrill® GI, product evaluations began at several hospitals in Europe and the US in the spring of 2024 and are ongoing. The evaluations have included a variety of tumor types, with positive outcomes reported in two published scientific papers (see below). The pictures shown below are a selection of high-quality core biopsies that BiBB’s team documented on site in examination rooms.
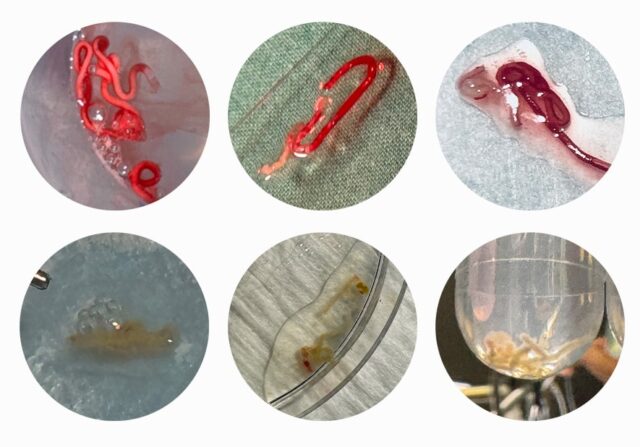
In January 2024, Dr. Antonio Mendoza Ladd, medical director of endoscopy at UC Davis Health in Sacramento, California, started a product evaluation of EndoDrill® GI. The results from the first eight patient cases have now been published in the article “Initial Experience With The Transmural Use Of A New Endoscopic Ultrasound Electric Core Needle Biopsy Device: A Case Series” in Endoscopy International Open1.
These patient cases represent the first transmural (through the wall of the gastrointestinal tract) biopsies using an EUS-CNB instrument (EndoDrill® GI) on tumors in the pancreas (n=5), retroperitoneum (n=2), and mediastinum (n=1). In all eight patient cases, a diagnosis was achieved using tissue biopsies taken with EndoDrill® GI (100% diagnostic accuracy) after a single needle pass. In four of these cases, patients had first undergone biopsies using manual EUS-FNA/FNB needle instruments (the current “gold standard”), which provided insufficient tissue samples for diagnosis. In all these cases, tissue samples obtained with EndoDrill® GI resulted in a complete diagnosis. The samples from EndoDrill® GI showed less blood contamination, fewer artifacts, and more intact tissue cores compared to what is typically seen with standard EUS-FNA/FNB instruments. The only noted adverse event was one case of mild bleeding, which was successfully controlled.
The authors’ impressions after the initial cases with EndoDrill® GI are that the tissue sampling method is effective, safe, and easy to install and use. They conclude by recommending a randomized clinical study comparing EndoDrill GI with standard EUS-FNA/FNB needle instruments to further assess the product’s efficacy and safety.
1 Mendoza Ladd A, Alsamman A, Meiklejohn K et al. Initial Experience With The Transmural Use Of A New Endoscopic Ultrasound Electric Core Needle Biopsy Device: A Case Series. Endoscopy International Open 2024. doi: 10.1055/a-2427-2311
Download the scientific article
The case series was also presented at a poster session at ACG 2024 in Philadelphia on Oct 29, 2024.Download the poster
A retrospective analysis conducted in the US and Europe was presented at ESGE Days in Barcelona on April 3, 2025. The study included data from the use of EndoDrill® GI for core tissue sampling in 28 patients across five university hospitals: Sacramento (USA), Bergen, Stockholm, Linköping, and Zagreb. The most common tumor locations were the pancreas, retroperitoneum, and stomach. In 22 of the 28 cases, a single needle puncture with EndoDrill® GI was sufficient to obtain diagnostic tissue samples.
The authors conclude: “The overall initial experience with this new EUS-CB device was positive. Our findings suggest that the device is effective and safe. Its effectiveness at obtaining adequate tissue with one pass could potentially shorten the overall duration of the procedure. Prospective studies comparing it to FNA/FNB needles will be required to further assess performance and safety.“
At The 4th Annual Michigan GI Society Conference in June 2025, Dr. Mohammed Barawi presented a clinical case series from Henry Ford St. John Hospital. Four patients – three with suspected liver disease and one with a suspected pancreatic tumor – underwent EUS-guided biopsy with EndoDrill® GI.
The powered 17G device provided diagnostic tissue samples in all cases, including one where previous sampling had been non-diagnostic. Dr. Barawi’s team highlighted both the diagnostic performance and procedural safety:
“The use of the EndoDrill device demonstrated strong diagnostic utility across an array of clinical presentations… EndoDrill yielded safe and effective results in this limited sample size.”
Reference: Oliver M, Kabir K, Ognjanovski E, Barawi M. (2025) Abstract presentation at the 4th Annual Michigan GI Society Conference.
A research team at Mayo Clinic in Jacksonville, Florida, has presented a poster from an ongoing precision medicine project using EndoDrill® GI to obtain core biopsies of pancreatic cancer (PDAC) for tissue slice cultures (ex vivo models). Initial results, based on three patients, showed that EndoDrill® GI can provide high-quality PDAC tissue cores with less blood contamination compared with manually operated standard needles (EUS-FNA/FNB). A larger study is planned to confirm reproducibility and further evaluate the potential of EndoDrill® GI as a new technique for tissue acquisition in advanced cancer research and future precision medicine applications.
Reference: Vera Garcia DV, Doeppler H, Pradieu G, Fluxa D, Osagiede O, Gomez V, Wagh MS, Brahmbhatt B, Kumbhari V, Storz P, Wallace MB. Drill2Culture – Establishing Ex Vivo PDAC Models via EndoDrill-Derived Core Biopsies Using EUS-Guided Biopsy. Poster presented at: Department of Medicine Research & Innovation Day, Mayo Clinic, Jacksonville, FL; 2025.
”This device will be a game changer in my opinion.”
Dr Antonio Mendoza Ladd MD, AGAF, FACG, FASGE Associate Professor of Medicine UC Davis Medical Director of Endoscopy UC Davis Health
Professor Fredrik Liedberg and his team at Skåne University Hospital are conducting an ongoing study programme focused on the urinary tract, which involves tissue sampling with EndoDrill® URO using a standard endoscope for muscle-invasive bladder cancer (MIBC)—i.e., tumours that have penetrated the bladder mucosa and muscle. A pilot clinical study involving ten patients was completed in 2022, and the results were published in the journal European Urology Open Science in June 2023. The study demonstrates that EndoDrill® URO can safely obtain treatment-determining samples earlier in the care chain when muscle-invasive bladder tumours are suspected. The research team concludes that there is reason to follow up the pilot study with a randomised efficacy study, which has been planned and received regulatory approval. The project also includes a health economic analysis.
The urologists’ long-term aim with the clinical programme is to demonstrate that early sampling with EndoDrill® URO can replace today’s standardised sampling as part of the TURBT procedure. The hypothesis is that the lead time from sampling to treatment initiation can be significantly reduced, potentially improving survival for this serious type of tumour.
”Histological verification and molecular classification of MIBC are possible for samples collected…”
(Eriksson P, et al., Urodrill – a novel MRI-guided endoscopic biopsy technique to sample and molecularly classify muscle-invasive bladder cancer without fractionating the specimen during transurethral resection, European Urology Open Science, Volume 53, July 2023, Pages 78-82)
The EDUX02 study demonstrates that EndoDrill® URO is the first endoscopic biopsy instrument that safely collects treatment-determining samples when bladder cancer is suspected.
BiBB’s IP portfolio is one of the company’s most critical assets. All of BiBB’s products have either been granted a patent or have a patent pending. The powered EndoDrill® system is protected by approved patents in USA (NoA), Europe, Japan, India and China and three pending international patent applications that have entered the national phase. In addition to patents, the EndoDrill® brand is registered in the major markets.
| Patent family | Patent pending | Approved patent | Valid until |
|---|---|---|---|
|
EndoDrill |
Patent family 1 |
||
|
|
|
Europe I | 2038 |
|
|
|
USA (NoA), Europe II, Japan, India, China | 2039 |
|
|
|
Europe III |
2039 |
|
|
Patent family 2
|
Japan |
2040 |
|
|
Patent family 3
|
China |
2041 |
|
EndoDrill (manual, Gen 1) |
USA, Canada | 2033 | |
|
Handle |
Europe, China | 2035 | |
|
EndoDrill Core Needle |
Sweden | 2038 | |
| EndoDrill® Trademark | USA | Europe, Australia, China, India, Japan, Canada |